Chemical bonds are the forces that hold atoms together in molecules and compounds. They result from the interactions of valence electrons between atoms. This documentation explores three primary types of chemical bonding: Ionic Bonding (Electron Transfer), Covalent Bonding (Electron Sharing), and Polar Covalent Bonding (Unequal Sharing).

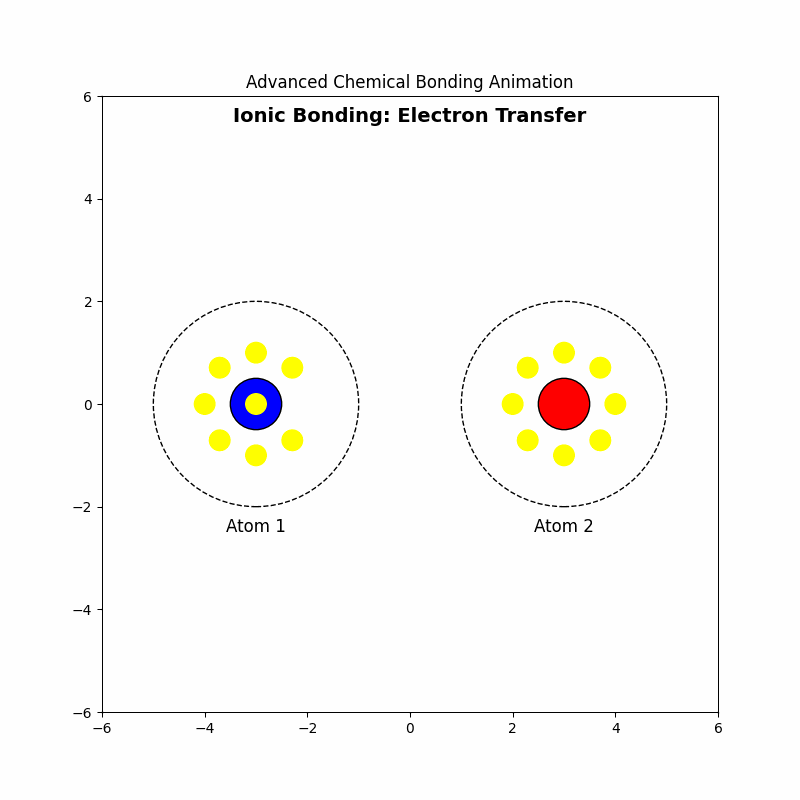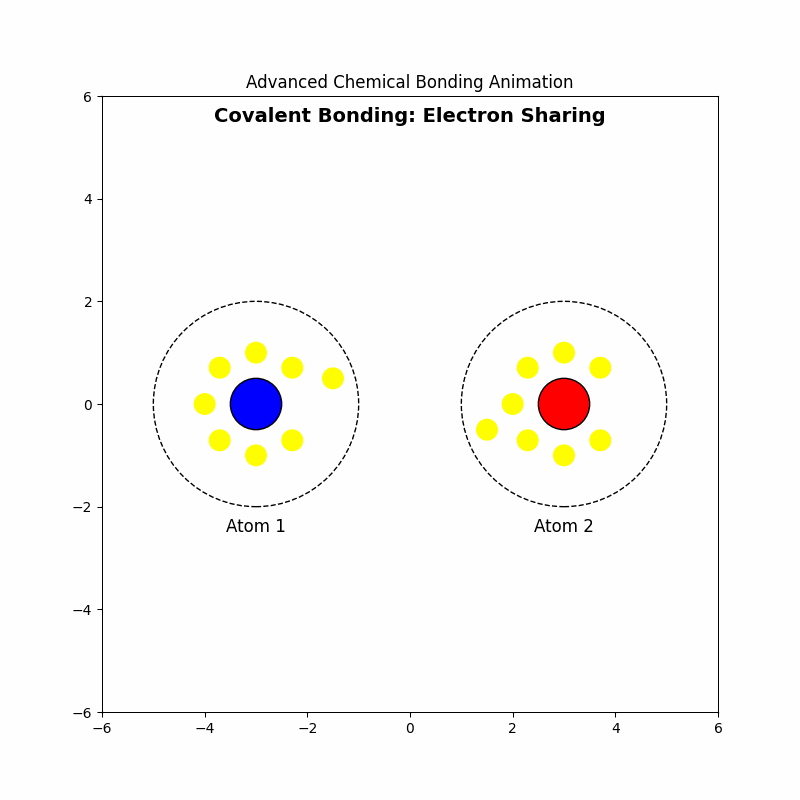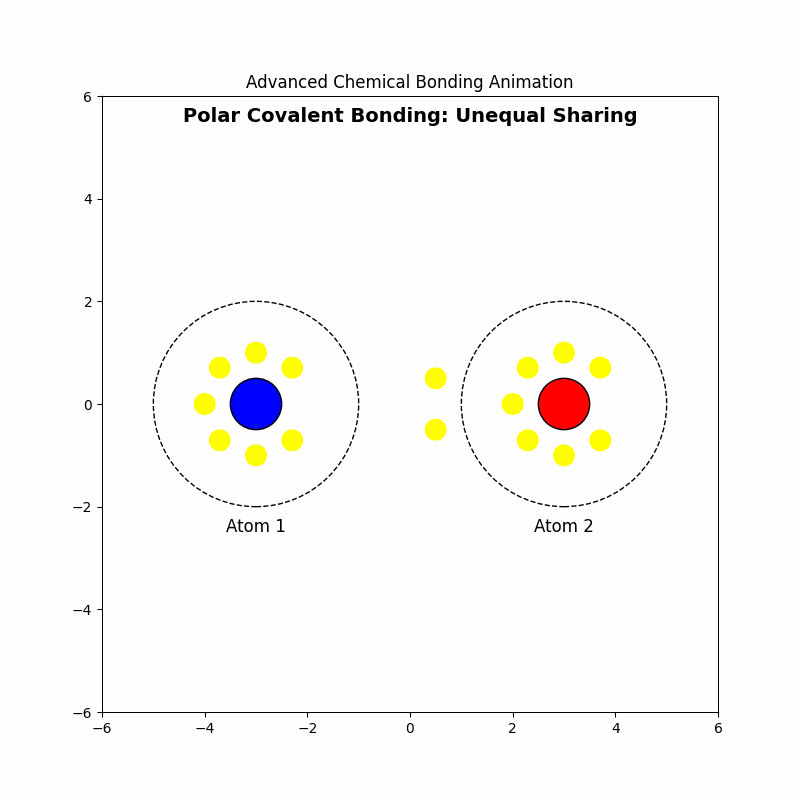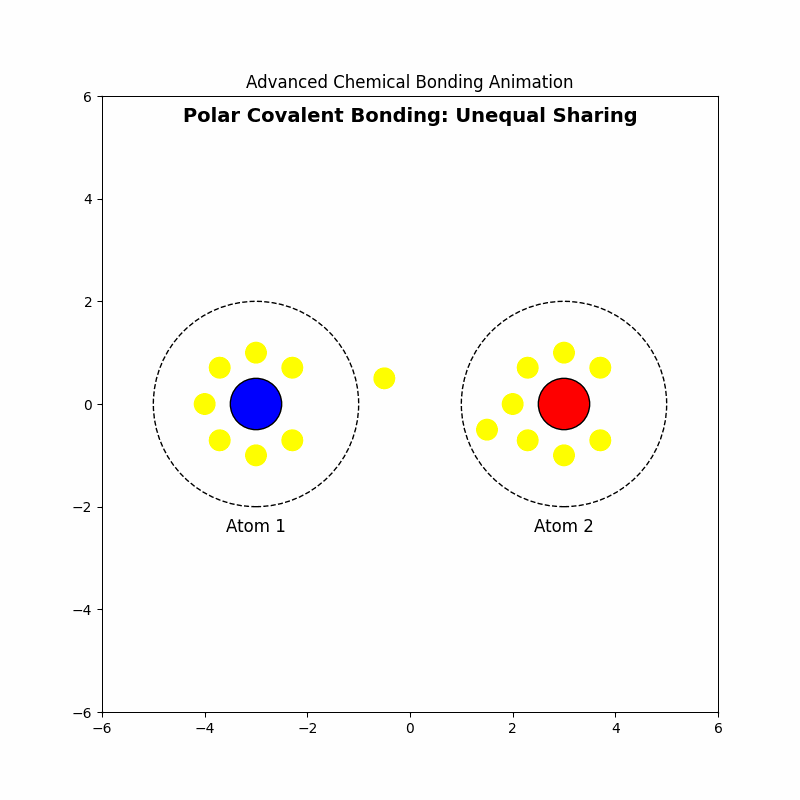
1. Ionic Bonding: Electron Transfer
🔗Ionic bonding occurs when electrons are transferred from one atom to another, resulting in the formation of ions. This typically happens between metals and nonmetals.
1.1 Formation of Ions
Atoms gain or lose electrons to achieve a stable noble gas electron configuration.
- Cations: Positively charged ions formed by the loss of electrons.
- Anions: Negatively charged ions formed by the gain of electrons.
1.2 Electrostatic Attraction
The ionic bond is the electrostatic force of attraction between oppositely charged ions.
Where:
- \( F \) is the force of attraction.
- \( k \) is Coulomb's constant.
- \( Q_1 \) and \( Q_2 \) are the charges of the ions.
- \( r \) is the distance between the ions.
1.3 Lattice Energy
Lattice energy is the energy released when gaseous ions form an ionic solid.
Where \( r_0 \) is the equilibrium distance between ions, and \( k' \) is a proportionality constant.
1.4 Example: Formation of Sodium Chloride (NaCl)
Sodium (Na) loses one electron to form \( \text{Na}^+ \), and chlorine (Cl) gains one electron to form \( \text{Cl}^- \). The ionic bond forms due to the attraction between \( \text{Na}^+ \) and \( \text{Cl}^- \).
2. Covalent Bonding: Electron Sharing
🤝Covalent bonding involves the sharing of electron pairs between atoms, allowing each atom to achieve a full valence shell. This type of bonding commonly occurs between nonmetal atoms.
2.1 Shared Electron Pairs
In a covalent bond, atoms share electrons to fill their valence shells:
- Single Bond: Sharing one pair of electrons (\( 2 \) electrons).
- Double Bond: Sharing two pairs of electrons (\( 4 \) electrons).
- Triple Bond: Sharing three pairs of electrons (\( 6 \) electrons).
2.2 Lewis Structures
Lewis structures represent covalent bonds using lines for shared electron pairs and dots for lone pairs.
Example: The Lewis structure of methane (\( \text{CH}_4 \)) shows carbon sharing electrons with four hydrogen atoms.
2.3 Bond Length and Energy
The bond length is the distance between the nuclei of two bonded atoms. Bond energy is the energy required to break the bond.
Shorter bonds are generally stronger and have higher bond energies.
2.4 Example: Formation of Oxygen Molecule (\( \text{O}_2 \))
Each oxygen atom shares two electrons, forming a double bond:
The double bond consists of one sigma (\( \sigma \)) bond and one pi (\( \pi \)) bond.
3. Polar Covalent Bonding: Unequal Sharing
⚖️Polar covalent bonding occurs when electrons are shared unequally between atoms due to differences in electronegativity.
3.1 Electronegativity Difference
Electronegativity (\( EN \)) is the ability of an atom to attract shared electrons. The bond polarity depends on the electronegativity difference (\( \Delta EN \)) between the atoms.
- Nonpolar Covalent Bond: \( \Delta EN \leq 0.4 \)
- Polar Covalent Bond: \( 0.4 < \Delta EN < 1.7 \)
- Ionic Bond: \( \Delta EN \geq 1.7 \)
3.2 Dipole Moments
Polar covalent bonds have a dipole moment (\( \mu \)), a measure of bond polarity:
Where:
- \( Q \) is the magnitude of the partial charges.
- \( r \) is the distance between the charges.
3.3 Partial Charges
The more electronegative atom acquires a partial negative charge (\( \delta^- \)), while the less electronegative atom acquires a partial positive charge (\( \delta^+ \)).
Representation:
3.4 Example: Formation of Hydrogen Chloride (HCl)
Chlorine is more electronegative than hydrogen:
- \( EN_{\text{Cl}} = 3.16 \)
- \( EN_{\text{H}} = 2.20 \)
- \( \Delta EN = 0.96 \) (Polar Covalent Bond)
Electrons are drawn closer to chlorine, creating a dipole moment.
Mathematical Explanation of Bonding Types
4.1 Ionic Bonding Energy Calculations
The energy of an ionic bond can be estimated using Coulomb's Law:
Example: Calculate the energy between \( \text{Na}^+ \) and \( \text{Cl}^- \) ions separated by \( r = 2.76 \, \text{Å} \).
- \( Q_1 = +1e \), \( Q_2 = -1e \)
- \( e = 1.602 \times 10^{-19} \, \text{C} \)
- \( k = 8.99 \times 10^9 \, \text{N m}^2/\text{C}^2 \)
This negative energy indicates an attractive force between the ions.
4.2 Covalent Bonding and Molecular Orbital Theory
In molecular orbital (MO) theory, atomic orbitals combine to form bonding and antibonding molecular orbitals.
For a hydrogen molecule (\( \text{H}_2 \)):
- Bonding MO: \( \sigma_{1s} = \psi_{1sA} + \psi_{1sB} \)
- Antibonding MO: \( \sigma_{1s}^* = \psi_{1sA} - \psi_{1sB} \)
Bond order is calculated as:
Where:
- \( N_b = 2 \) (number of electrons in bonding orbitals)
- \( N_a = 0 \) (number of electrons in antibonding orbitals)
A bond order of 1 indicates a single covalent bond.
4.3 Polar Covalent Bonding and Electronegativity
Electronegativity values quantify an atom's ability to attract shared electrons. The greater the difference, the more polar the bond.
Pauling's equation for estimating bond energy (\( D_{AB} \)) based on electronegativity differences:
Where:
- \( D_{AA} \) and \( D_{BB} \) are the bond energies of the homonuclear diatomic molecules \( A_2 \) and \( B_2 \).
- \( \chi_A \) and \( \chi_B \) are the electronegativities of atoms \( A \) and \( B \).
Visual Representations
5.1 Ionic Lattice Structure
In ionic compounds like NaCl, ions arrange in a crystal lattice to maximize attraction and minimize repulsion. This structure contributes to the high melting and boiling points of ionic compounds.
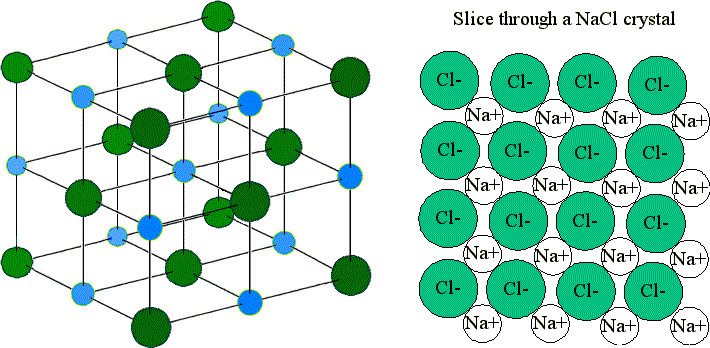
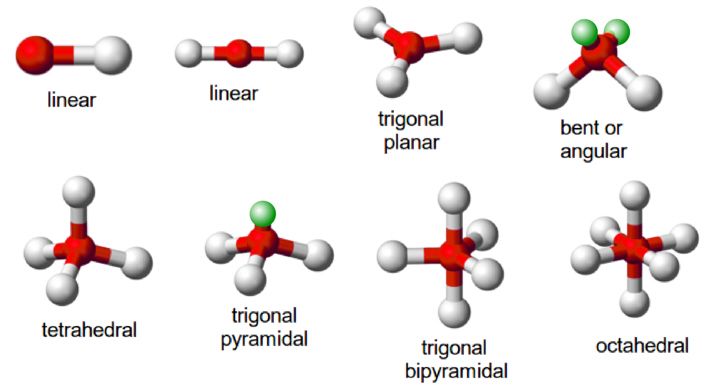
5.2 Covalent Molecule Shapes
Valence Shell Electron Pair Repulsion (VSEPR) theory predicts molecular geometry based on electron pair repulsion.
- Linear: \( \text{CO}_2 \) (180° bond angle)
- Tetrahedral: \( \text{CH}_4 \) (109.5° bond angle)
- Trigonal Pyramidal: \( \text{NH}_3 \) (approx. 107° bond angle)
- Bent: \( \text{H}_2\text{O} \) (approx. 104.5° bond angle)
5.3 Polar Molecules and Dipoles
Polar molecules have a net dipole moment due to asymmetrical charge distribution caused by polar bonds and molecular geometry.
Example: Water (\( \text{H}_2\text{O} \)) is polar because of its bent shape and the electronegativity difference between hydrogen and oxygen.

Applications and Importance
6.1 Biological Systems
Chemical bonding is crucial in biological molecules:
- DNA Structure: Hydrogen bonds between nucleotide bases hold the two strands of the DNA double helix together.
- Protein Folding: Ionic bonds, hydrogen bonds, and disulfide bridges stabilize the three-dimensional structures of proteins.
6.2 Materials Science
Understanding bonding leads to the development of new materials with specific properties:
- Ceramics: Ionic and covalent bonds provide high melting points and hardness.
- Polymers: Covalent bonds form long-chain molecules with versatile applications in plastics and fibers.
- Semiconductors: Covalent bonding in silicon crystals is essential for electronic devices.
6.3 Chemical Reactions
Bond formation and breaking are fundamental to chemical reactions:
- Exothermic Reactions: Release energy when new bonds form, such as combustion.
- Endothermic Reactions: Absorb energy to break bonds, such as photosynthesis.
Conclusion
Understanding ionic, covalent, and polar covalent bonding is essential for comprehending the structure and behavior of matter. These bonds result from the interactions of electrons between atoms, leading to the vast diversity of substances in the universe.
The principles of electron transfer, sharing, and unequal sharing explain why elements form certain types of bonds and how these bonds affect the properties of compounds. Mastery of these concepts is fundamental in chemistry, biology, physics, and materials science.
Advancements in technology and science continue to build upon our knowledge of chemical bonding, leading to new discoveries and innovations that enhance our understanding of the natural world.
import matplotlib.pyplot as plt
from matplotlib.animation import FuncAnimation
import numpy as np
# Parameters
frames = 200 # Number of frames for animation
shell_radius = 2.0
electron_radius = 0.2
animation_speed = 50 # Speed of animation (ms between frames)
# Create the figure and axis
fig, ax = plt.subplots(figsize=(8, 8))
ax.set_xlim(-6, 6)
ax.set_ylim(-6, 6)
ax.set_aspect('equal')
ax.set_title('Advanced Chemical Bonding Animation')
# Atoms with nucleus and electron shells
atom1_nucleus = plt.Circle((-3, 0), 0.5, color='blue', ec='black', label='Atom 1')
atom1_shell = plt.Circle((-3, 0), shell_radius, color='none', ec='black', linestyle='--')
atom2_nucleus = plt.Circle((3, 0), 0.5, color='red', ec='black', label='Atom 2')
atom2_shell = plt.Circle((3, 0), shell_radius, color='none', ec='black', linestyle='--')
# Adding atoms and shells to the plot
ax.add_artist(atom1_nucleus)
ax.add_artist(atom1_shell)
ax.add_artist(atom2_nucleus)
ax.add_artist(atom2_shell)
# Initial positions of valence electrons around Atom 1 and Atom 2
atom1_electrons = [plt.Circle((-3 + np.cos(theta), np.sin(theta)), electron_radius, color='yellow', ec='black')
for theta in np.linspace(0, 2 * np.pi, 8, endpoint=False)]
atom2_electrons = [plt.Circle((3 + np.cos(theta), np.sin(theta)), electron_radius, color='yellow', ec='black')
for theta in np.linspace(0, 2 * np.pi, 8, endpoint=False)]
# Adding electrons to the plot
for electron in atom1_electrons + atom2_electrons:
ax.add_artist(electron)
# Annotations for bond types
annotation = ax.text(0, 5.5, '', fontsize=14, ha='center', fontweight='bold')
atom1_label = ax.text(-3, -2.5, 'Atom 1', fontsize=12, ha='center')
atom2_label = ax.text(3, -2.5, 'Atom 2', fontsize=12, ha='center')
# Initialization function
def init():
for electron in atom1_electrons + atom2_electrons:
electron.set_color('yellow')
annotation.set_text('')
return atom1_electrons + atom2_electrons + [annotation]
# Animation function for bonding
def animate(i):
# Phase 1: Ionic Bonding (First 70 frames)
if i < frames // 3:
move_fraction = i / (frames // 3)
atom1_electrons[0].center = (-3 + 6 * move_fraction, 0)
annotation.set_text('Ionic Bonding: Electron Transfer')
# Phase 2: Covalent Bonding (Next 70 frames)
elif i < 2 * frames // 3:
move_fraction = (i - frames // 3) / (frames // 3)
atom1_electrons[0].center = (-1.5 + 3 * move_fraction, 0.5)
atom2_electrons[0].center = (1.5 - 3 * move_fraction, -0.5)
annotation.set_text('Covalent Bonding: Electron Sharing')
# Phase 3: Polar Covalent Bonding (Last 60 frames)
else:
move_fraction = (i - 2 * frames // 3) / (frames // 3)
atom1_electrons[0].center = (0.5 - move_fraction, 0.5)
atom2_electrons[0].center = (0.5 + move_fraction, -0.5)
annotation.set_text('Polar Covalent Bonding: Unequal Sharing')
return atom1_electrons + atom2_electrons + [annotation]
# Create the animation
anim = FuncAnimation(fig, animate, init_func=init, frames=frames, interval=animation_speed, blit=True)
# Save the animation as a GIF
anim.save('advanced_fast_chemical_bonding_animation.gif', writer='pillow')
# Display the final frame of the plot
plt.show()